- All participants were on conventional therapy, then were assigned to take either YORVIPATH or placebo
- Doses for YORVIPATH, placebo, and conventional therapy were adjusted by a doctor based on blood calcium levels
Results With YORVIPATH
YORVIPATH was assessed in a 26-week clinical study
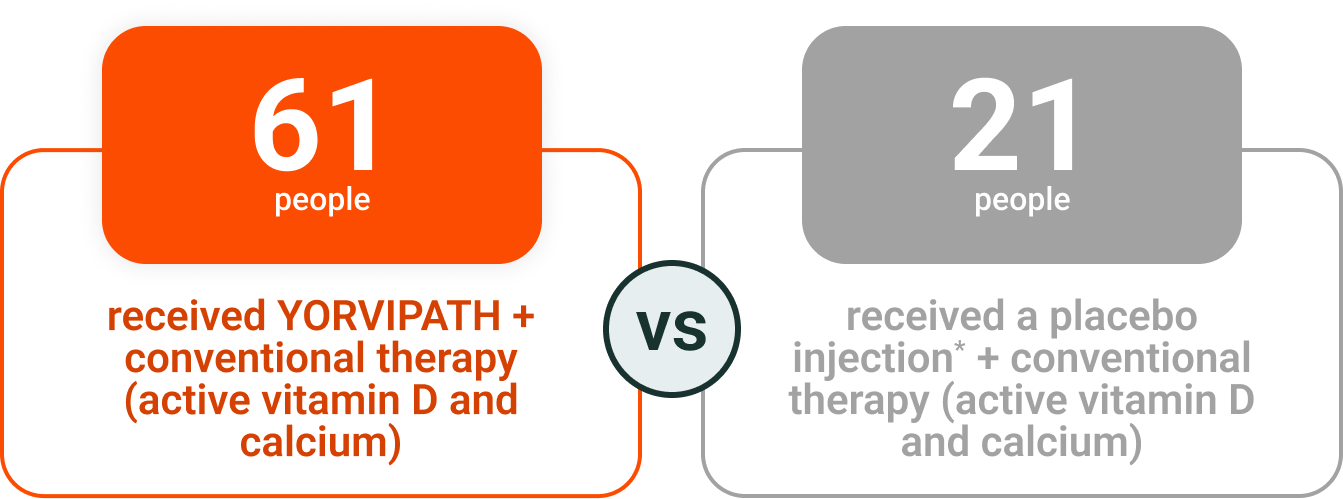

The effectiveness of YORVIPATH was measured by a strict set of treatment criteria
Nearly 70% of people responded to treatment with YORVIPATH at Week 26‡
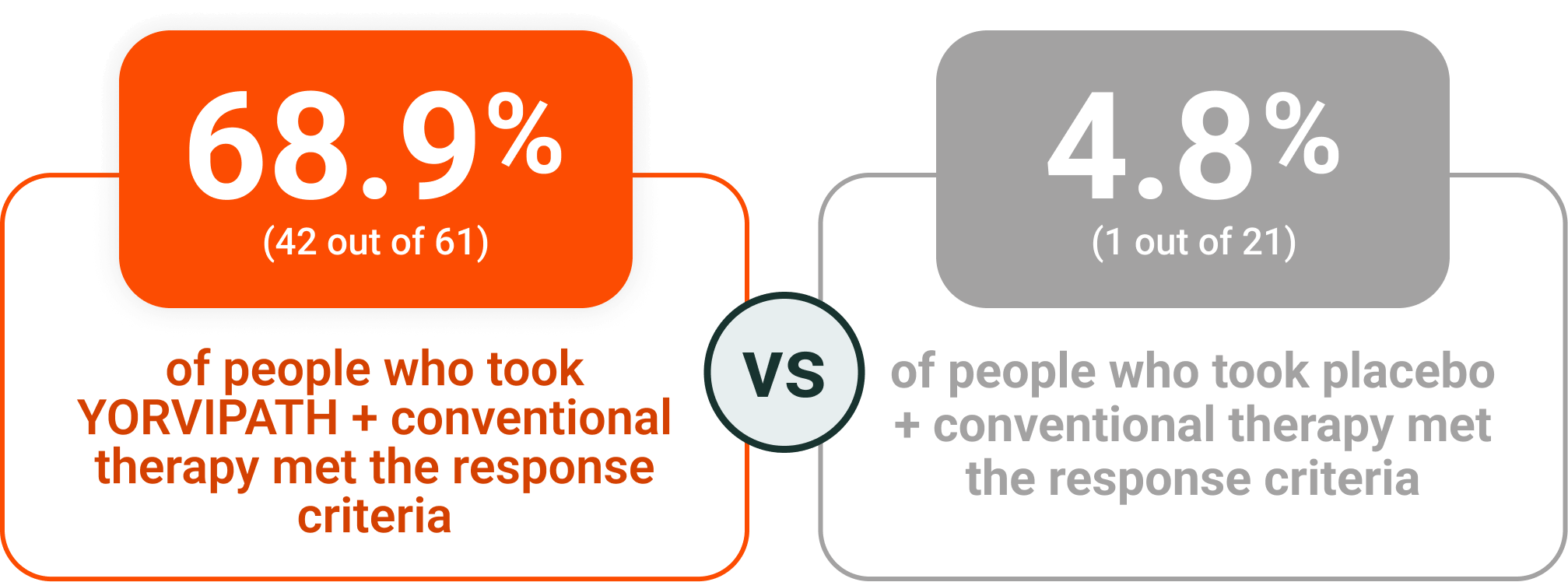
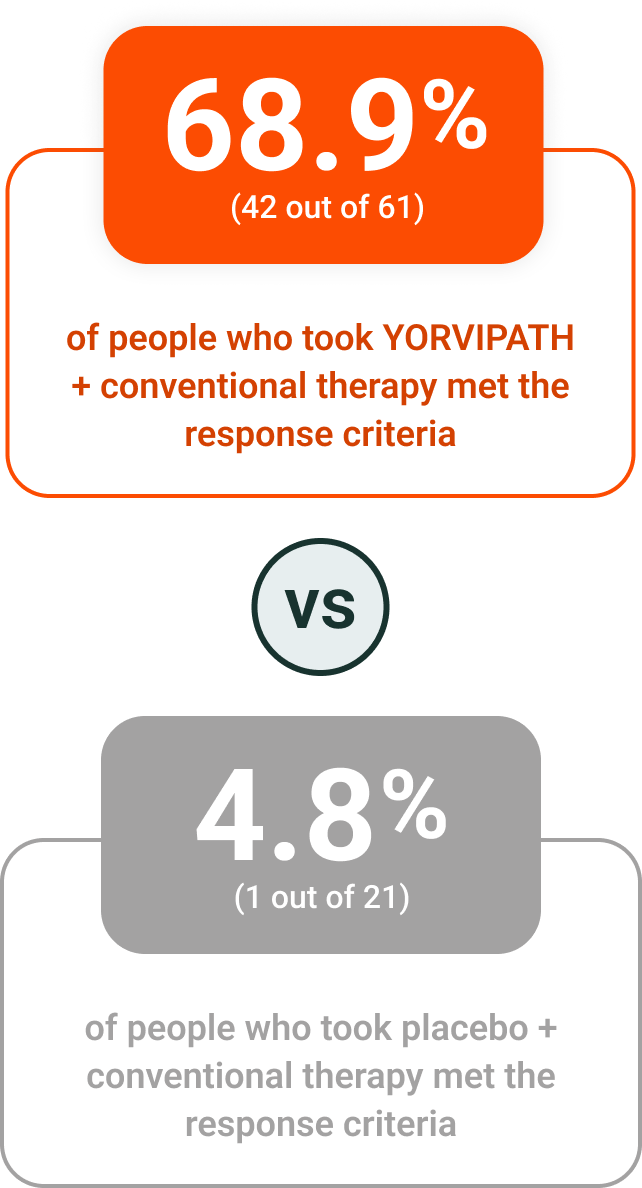
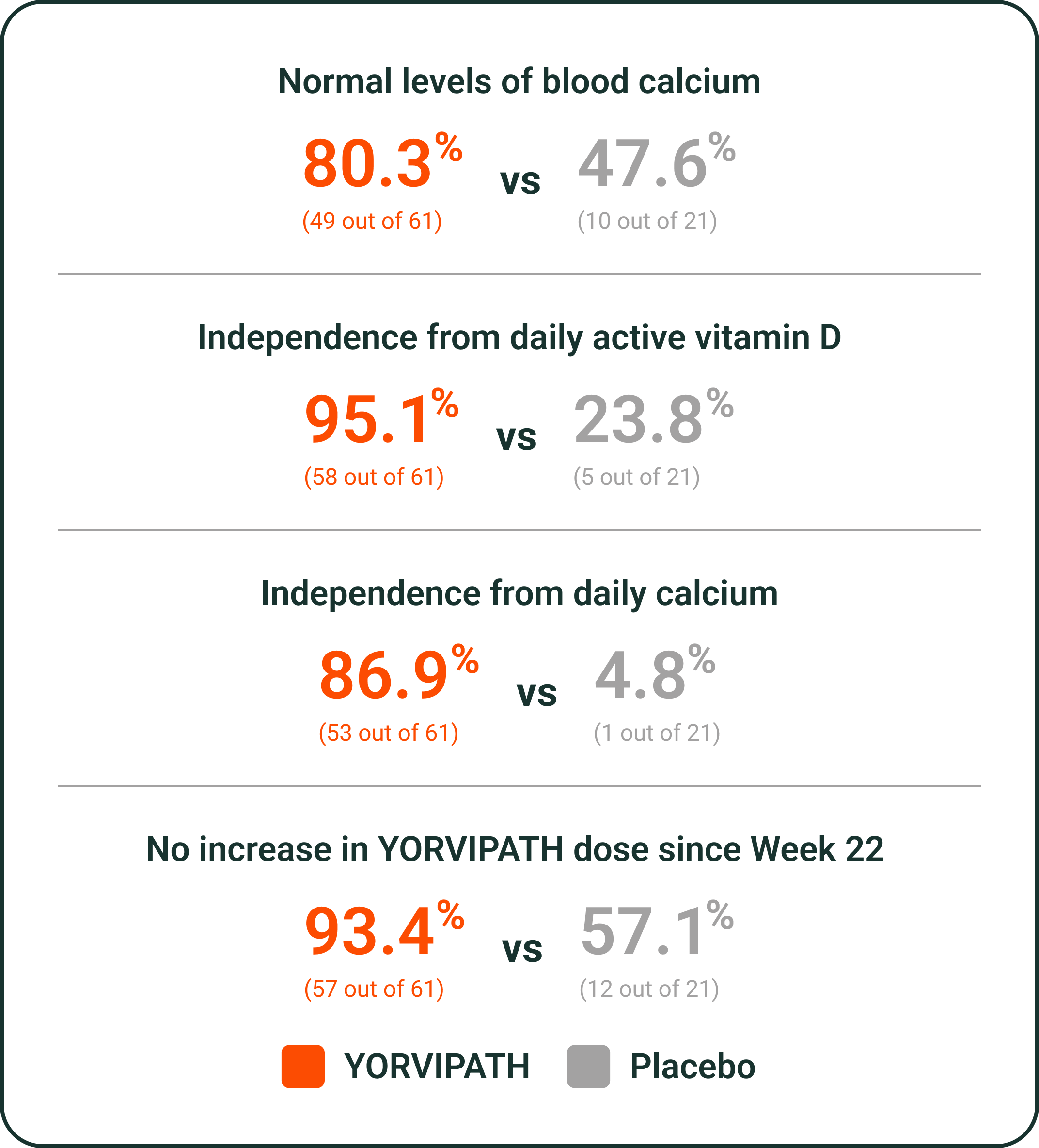
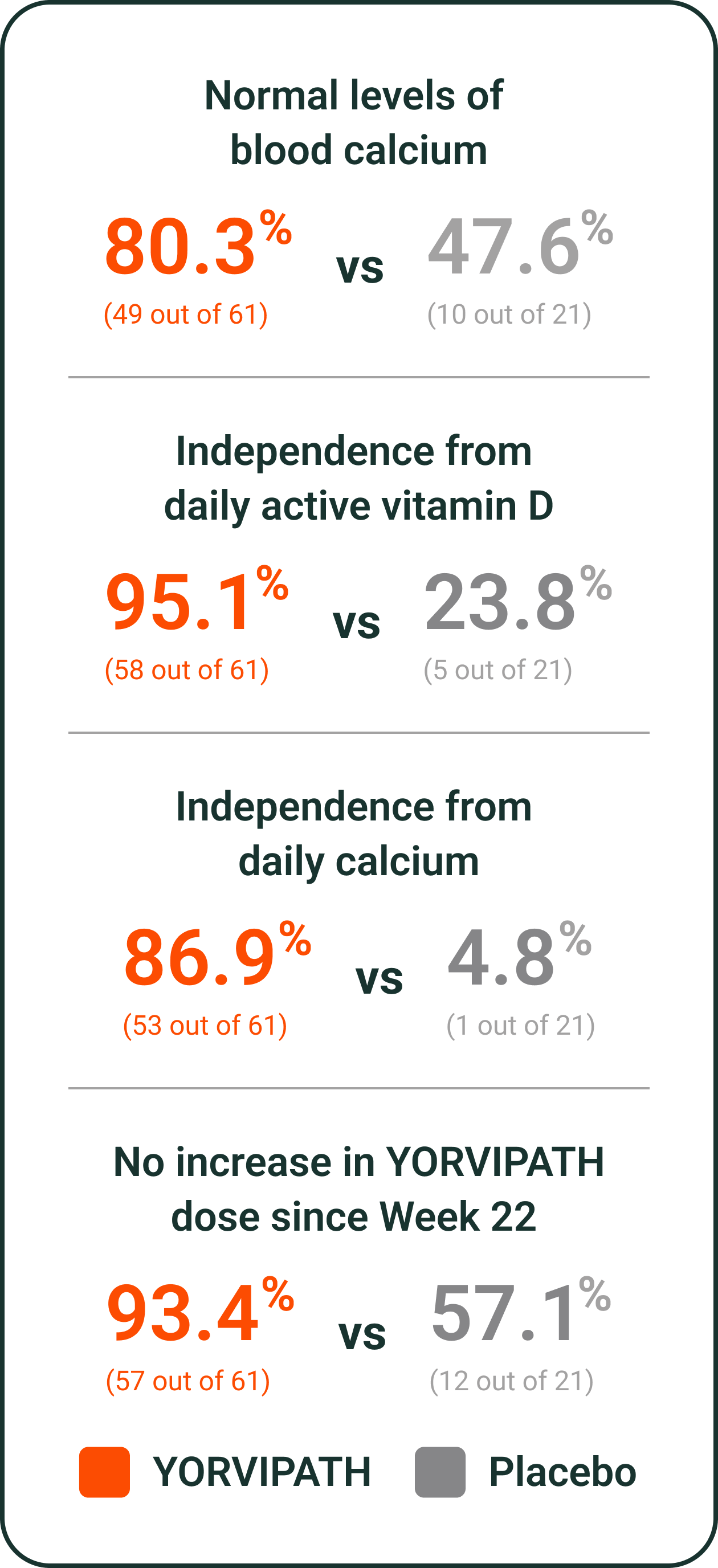
YORVIPATH helped to reduce the amount of calcium and active vitamin D people needed daily‖
Reduction in the number of daily active vitamin D and calcium pills in the YORVIPATH study
Stay connected
Fill out the form below for important information and resources about YORVIPATH.